Search
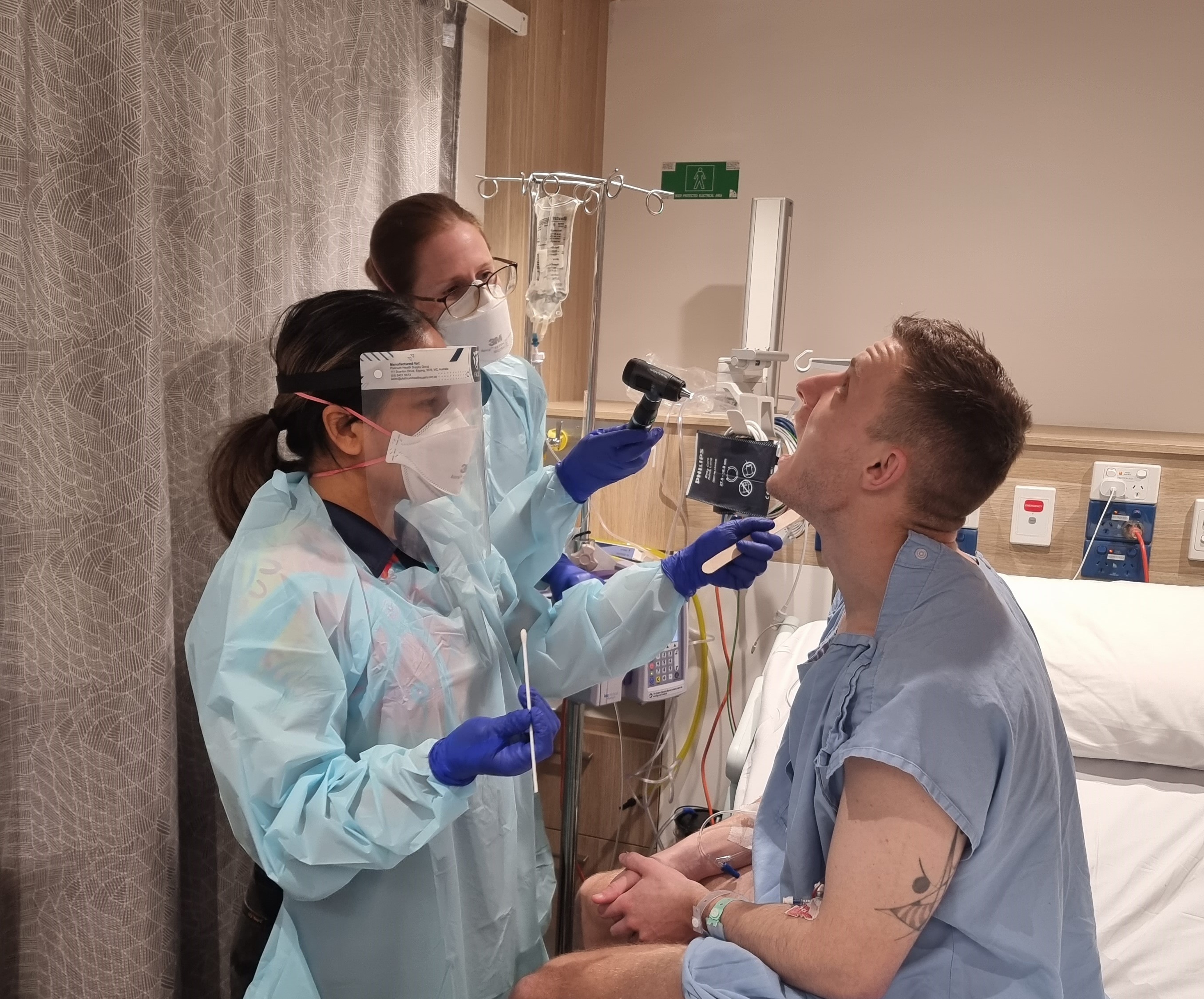
A unique study purposely giving participants Streptococcus pyogenes (Strep A) to learn how much penicillin it takes to prevent infection has found the amount needed is much lower than previously thought – a discovery that will transform thinking on treatment for people living with rheumatic heart disease (RHD).

Two infectious disease researchers from Papua New Guinea (PNG) dedicated to reducing rates of childhood mortality in their home country are making significant advances thanks to support from the Deborah Lehmann Research Award (DLRA).

A life-saving meningococcal vaccine covering all five common strains of the deadly disease could soon be available thanks to vital research demonstrating the safety and effectiveness of a combination Men ABCWY vaccine.

Researchers from the Wesfarmers Centre of Vaccines and Infectious Diseases, based at The Kids Research Institute Australia, have launched an online guidance tool designed to help families and health-care providers in WA learn the best way to protect babies and young children against life-threatening respiratory syncytial virus (RSV).

Two projects led by The Kids Research Institute Australia have been awarded more than $2.5 million to fund innovative ideas focused, respectively, on combating persistent ear infections and investigating how dangerous fungi invade the bodies of immunocompromised people.

We honour the memory of Emeritus Professor Michael Alpers, a colleague and friend to many at The Kids Research Institute Australia, who passed away on December 3, 2024.

Congratulations to six researchers from The Kids Research Institute Australia, who will use valuable support from the Raine Medical Research Foundation’s 2024 grant round to undertake projects focused on improving the health and wellbeing of babies, children and young people.
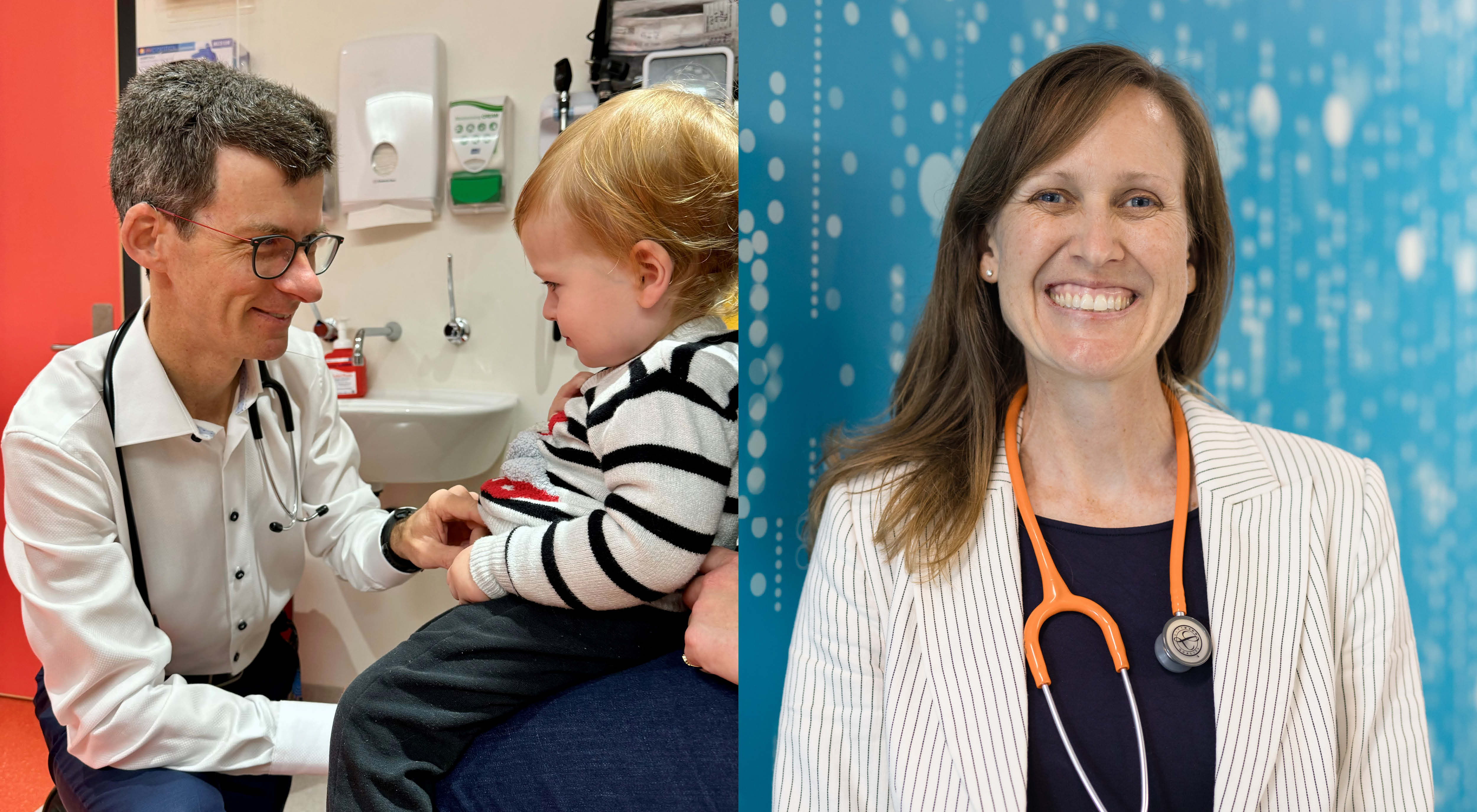
Two Perth clinician-scientists have been recognised as national leaders in infectious disease research after being elected as Fellows of the esteemed Australian Academy of Health and Medical Sciences.

The Wesfarmers Centre of Vaccines and Infectious Diseases (WCVID) awarded three successful recipients with Catalyst research grants, with each researcher receiving $80,000 towards their chosen project.

One out of every 10 children with a bloodstream infection are infected with a multi-drug resistant organism in the nation’s first-ever surveillance study investigating the prevalence of paediatric antimicrobial resistance (AMR).
