Search
Impaired oxygen delivery or blood flow to the brain around the time of birth can cause injury. Hypoxic ischaemic encephalopathy is a leading cause of death and disability in term and near-term infants.
Skin care for very and extremely preterm infant is an important and previously underappreciated topic. Coconut oil skin care for preterm infants is a promising option, but several important questions remain including the theoretical potential for allergic sensitization.
Viral infections are associated with significant morbidity and mortality in neonates. The COVID-19 pandemic led to changes in viral epidemiology in Western Australia. The impact on patients in neonatal intensive care is uncertain.
Sepsis is one of the leading causes of neonatal mortality. There is heterogeneity in the outcomes measured and reported in studies of neonatal sepsis. To address this challenge, a core outcome set (COS) for research on neonatal sepsis was needed.
Tobias Strunk MD, PhD, FRACP Head, Neonatal Health tobias.strunk@thekids.org.au Head, Neonatal Health Clinical Professor Tobias Strunk is a
Tobias Strunk MD, PhD, FRACP Head, Neonatal Health tobias.strunk@thekids.org.au Head, Neonatal Health Clinical Professor Tobias Strunk is a
Tobias Strunk MD, PhD, FRACP Head, Neonatal Health tobias.strunk@thekids.org.au Head, Neonatal Health Clinical Professor Tobias Strunk is a
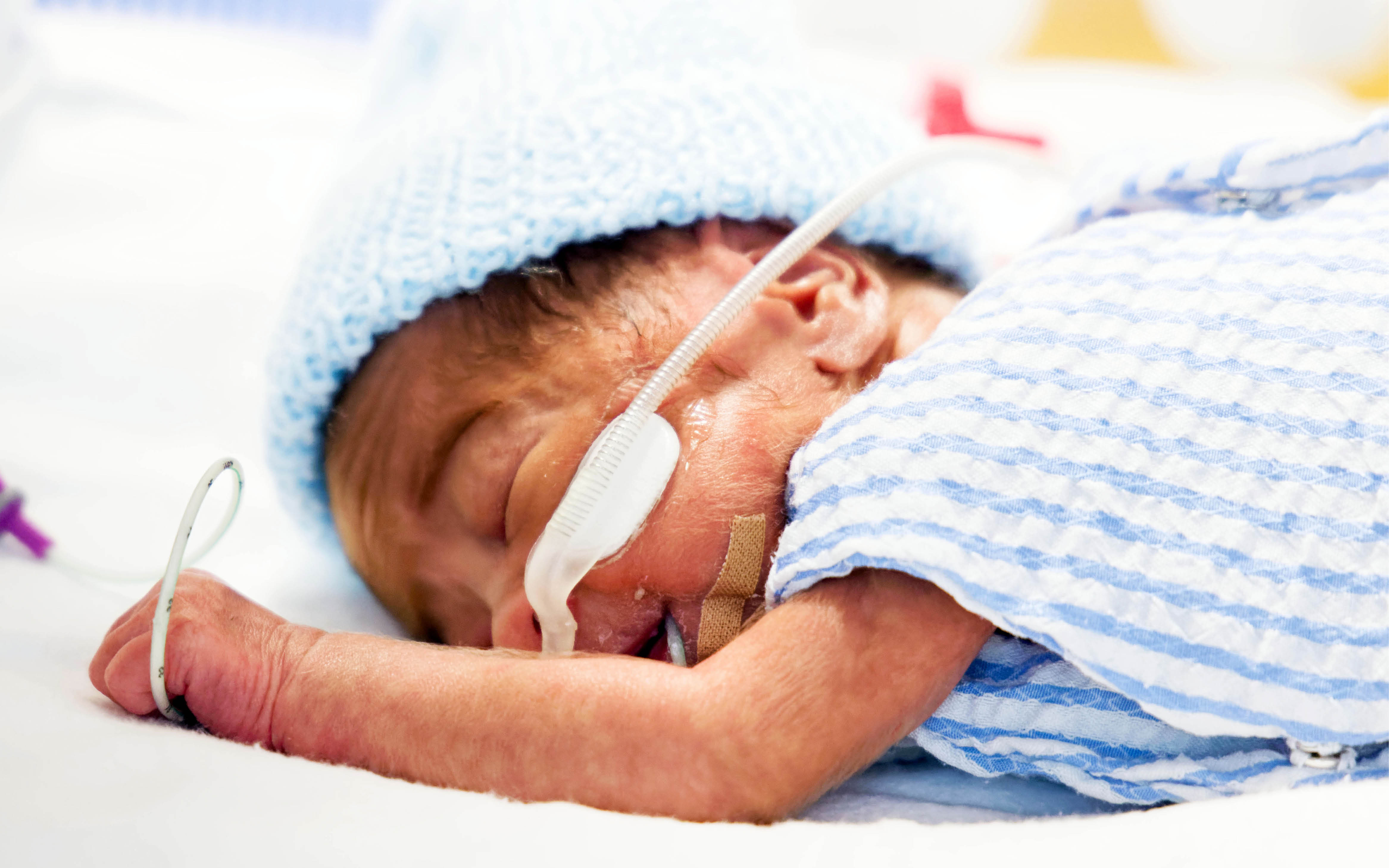
Preterm babies have a heightened risk of infection as their immune system is not mature. The Neonatal Health Team is exploring new ways to diagnose, prevent and treat infections in WA's smallest patients .
Delayed cord clamping (DCC) is an evidence-based intervention that reduces mortality, anaemia and disability in infants born <37 weeks' gestation who do not require immediate resuscitation. However, it is neither reliably recorded nor routinely implemented in Australia. The Wait a Minute or More study aims to reduce this gap between the evidence and practice by integrating timely sharing of cord clamping data with Evidence-based Practice for Improving Quality methods to increase the proportion of preterm infants receiving DCC for 60s or longer (DCC60).
While a systematic review exists detailing neonatal sepsis outcomes from clinical trials, there remains an absence of a qualitative systematic review capturing the perspectives of key stakeholders.
