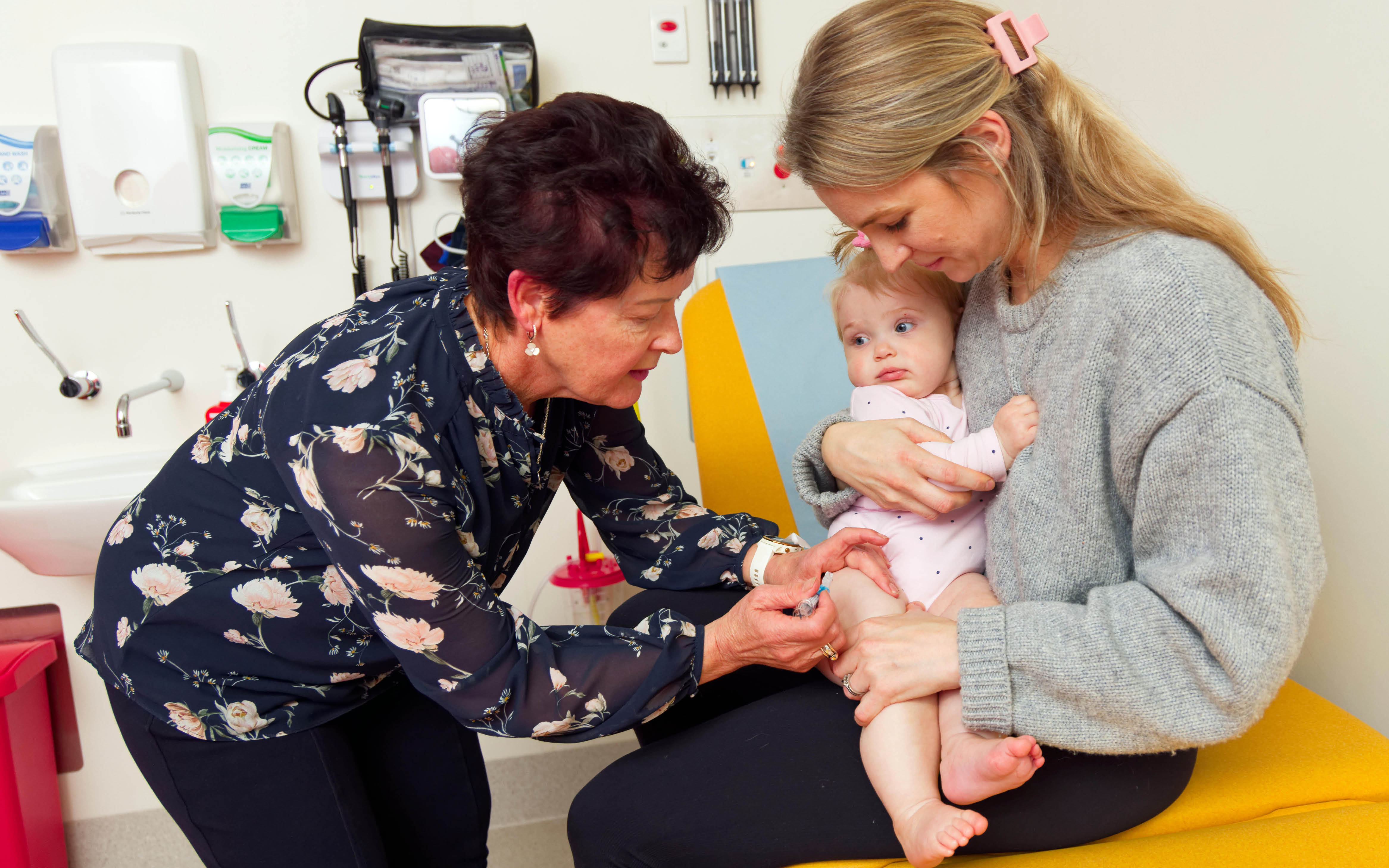
The Vaccine Trials Group (VTG) team of doctors, nurses, researchers, scientists, students and phlebotomists is testing and evaluating the safety and effectiveness of new vaccines for a range of diseases, as well as monitoring vaccines.
The VTG was established in 2000 to provide a coordinated approach to the development, delivery, assessment and promotion of vaccines, infectious disease and allergy treatments in the community. The team plays a vital role in the development and evaluation of vaccines recommended for children and adults throughout Australia.
The VTG leads national studies focusing on a range of diseases including influenza,
pneumococcal, meningococcal, respiratory syncytial virus (RSV) and whooping cough.
Team leader

MBBS MRCP(UK) FRACP
Head, Vaccine Trials Group
Team members (16)
Clinical Research Manager

BSc BMedSc(hons) PhD
Honorary Research Associate

Zoe Ellis
Clinical Laboratory Coordinator

Camille Gibson
Research Nurse

Jan Jones
Laboratory Research Assistant

Karen Jones
Executive Assistant

Sonia McAlister
Postdoctorial Researcher

Kieran Veale
Research Nurse

Ushma Wadia
Clinical Research Fellow

Miriam West
Research Nurse

Kerrie Lisgaris
Research Nurse

Jonah Macliver
Laboratory Research Assistant

Shania Tansil
Laboratory Research Assistant

Chantalia Tedja
Laboratory Research Assistant

Madeline Ong
Clinical Research Fellow

Julia Kets
Research Nurse
Featured projects
BCG vaccination to Reduce the impact of COVID-19 in Australian healthcare workers following Coronavirus Exposure (BRACE) Trial
ATOMIC Ears: A Phase IIB randomised controlled trial to assess safety, tolerability and acceptability of a 5-day Dornase alfa treatment as an adjunct therapy to ventilation tube insertion for otitis media in children
Other projects
Sore Throat Melbourne and Perth Study: The Australian Strep A Vaccine Initiative (ASAVI) Urban Pharyngitis Surveillance Study (STAMPS) COVALIA (COVid vaccine trial for austrALIA): A phase I, double-blind, dose-ranging, randomised, placebo-controlled trial to study the safety and immunogenicity of a DNA-based vaccine against COVID-19 (COVIGEN) in healthy participants aged 18 to 75 years o A phase IIIB, randomized, controlled, observer-blind study to evaluate safety and immunogenicity of GSK’s meningococcal ABCWY vaccine when administered in healthy adolescents and adults, previously primed with meningococcal ACWY vaccine (BOOST) A phase 3, randomized, double-blinded, placebo-controlled trial toevaluate the efficacy and safety of a respiratory syncytial virus (RSV) prefusion F subunit vaccine in infants born to women vaccinated during pregnancy (Matisse) Safety and Immunogenicity of Early Quadrivalent Influenza Vaccine: A phase 2prospective randomised open-label feasibility study (FluBub) A Phase 3, randomized, double-blind trial to evaluate the safety and immunogenicity of a 20-valent pneumococcal conjugate vaccine given as a series of 2 infant doses and 1 toddler dose in healthy infants (NeXXstep) All Vaccine Trials Group projects